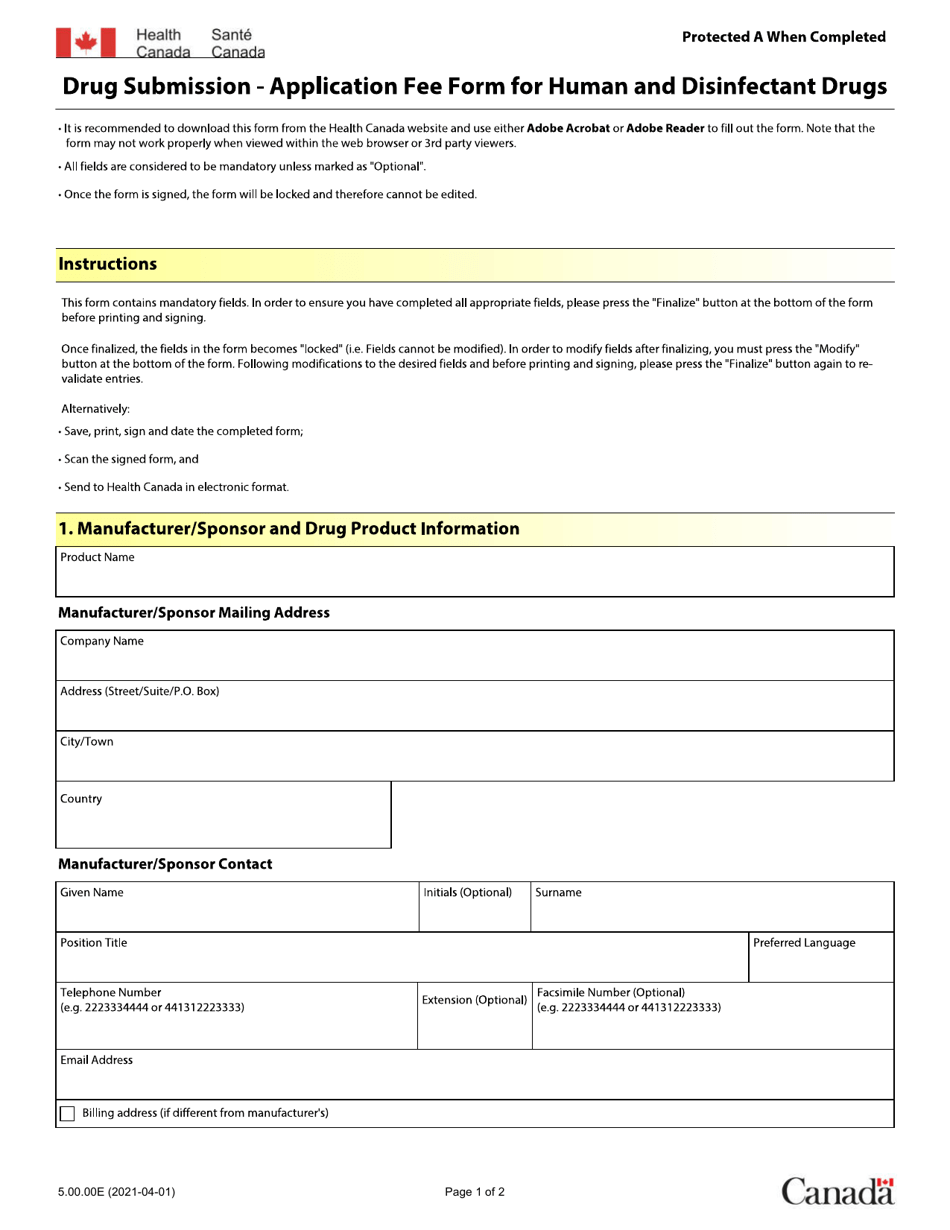
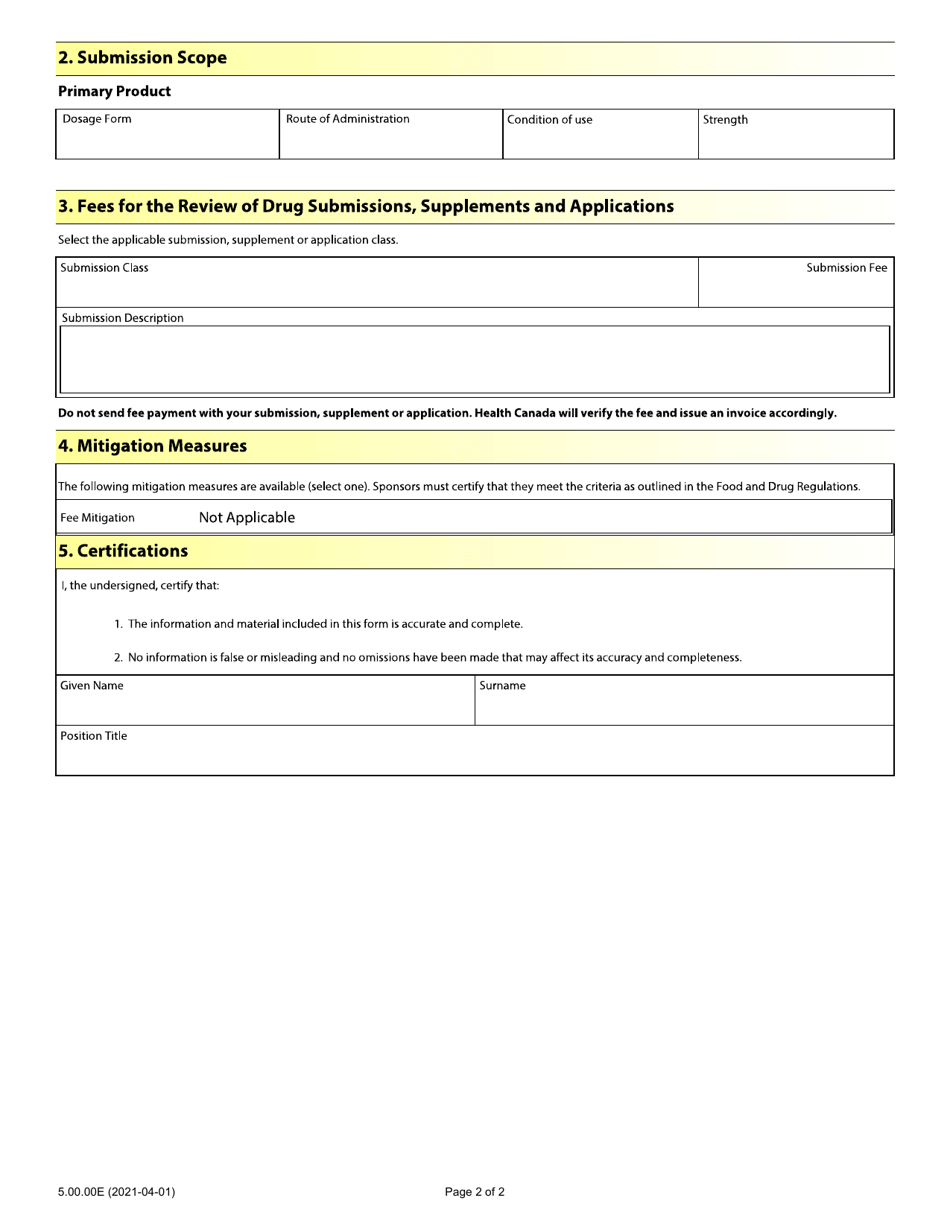
Form 5.00.00E Drug Submission - Application Fee Form for Human and Disinfectant Drugs - Canada
Form 5.00.00E Drug Submission - Application Fee Form for Human and Disinfectant Drugs is used in Canada for submitting applications and paying the required fees for the approval of human and disinfectant drugs.
The applicant or sponsor of the drug files the Form 5.00.00E Drug Submission - Application Fee Form for Human and Disinfectant Drugs in Canada.
FAQ
Q: What is Form 5.00.00E?
A: Form 5.00.00E is the Drug Submission - Application Fee Form for Human and Disinfectant Drugs in Canada.
Q: What is the purpose of Form 5.00.00E?
A: The purpose of Form 5.00.00E is to collect the required application fees for the review of drug submissions in Canada.
Q: What type of drugs does Form 5.00.00E apply to?
A: Form 5.00.00E applies to both human drugs and disinfectant drugs.
Q: Who needs to fill out Form 5.00.00E?
A: Anyone submitting a drug submission for review in Canada needs to fill out Form 5.00.00E.
Q: What information is required on Form 5.00.00E?
A: Form 5.00.00E requires information such as the applicant's name, contact information, and the type of drug being submitted.
Q: Are there any fees associated with Form 5.00.00E?
A: Yes, there are application fees associated with Form 5.00.00E, which are based on the type of drug submission.
Q: How should Form 5.00.00E be submitted?
A: Form 5.00.00E should be submitted along with the required application fees to the appropriate regulatory authority in Canada.
ADVERTISEMENT
Download Form 5.00.00E Drug Submission - Application Fee Form for Human and Disinfectant Drugs - Canada
4.6 of 5 ( 8 votes )

1

2
Prev 1 2 Next
ADVERTISEMENT
Linked Topics
Health Canada Canadian Federal Legal Forms Canada Legal Forms Legal

Related Documents
- Submission Certification for Human and Disinfectant Drugs - Canada
- Submission Certificate for New Drug Submissions (Nds), Supplement to a New Drug Submission (Snds), Supplement Abbreviated New Drug Submissions (Sands), Abbreviated New Drug Submissions (Ands), Notifiable Changes (Nc) - Canada
- Submission Certificate for a New Drug Submission (Nds), Supplement to a New Drug Submission (Snds), Supplement to an Abbreviated New Drug Submission (Sands), Abbreviated New Drug Submission (Ands), or Notifiable Change (Nc) - Canada
- Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application/Attestation - Canada
- Drug Submission - Application Fee Form for Human and Disinfectant Drugs - Canada
- Pre-submission Meeting Request - Veterinary Drugs Directorate - Health Products and Food Branch (Hpfb) - Canada
- Veterinary Drug Submission Application and Fee Form - Canada, 2024
- Form HC3011 Drug Submission Application Form for: Human, Veterinary or Disinfectant Drugs and Clinical Trial Application/Attestation - Canada
- Form 5.00.02E Submission Certificate for a New Drug Submission (Nds), Supplement to a New Drug Submission (Snds), Supplement to an Abbreviated New Drug Submission (Sands), Abbreviated New Drug Submission (Ands), or Notifiable Change (Nc) - Canada
- Master File (Mf) Application Fee Form for Human Drugs - Canada
- Form 4.11E Master File (Mf) Application Fee Form for Human Drugs - Canada
- Form 4.16E Master File (Mf) Application Fee Form for Human Drugs - Canada
- Form 4.12E Master File (Mf) Application Fee Form for Human Drugs - Canada
- Form 4.19E Master File (Mf) Application Fee Form for Human Drugs - Canada
- Advance Payment Details for Master Files for Human and Disinfectant Drugs, and Certificate of Supplementary Protection Applications - Canada
- Administrative Changes - Certification Form for Human and/or Disinfectant Drug Submissions and Applications - Canada
- Sponsor Attestation Checklist for Abbreviated New Drug Submissions (Andss) - Canada
- Appendix C Request for Human Drugs Reconsideration Template - Canada
- Convert Word to PDF
- Convert Excel to PDF
- Convert PNG to PDF
- Convert GIF to PDF
- Convert TIFF to PDF
- Convert PowerPoint to PDF
- Convert JPG to PDF
- Convert PDF to JPG
- Convert PDF to PNG
- Convert PDF to GIF
- Convert PDF to TIFF
- Split PDF
- Merge PDF
- Sign PDF
- Compress PDF
- Rearrange PDF Pages
- Make PDF Searchable
- About
- Help
- DMCA
- Privacy Policy
- Terms Of Service
- Contact Us
- All Topics
Legal Disclaimer: The information provided on TemplateRoller.com is for general and educational purposes only and is not a substitute for professional advice. All information is provided in good faith, however, we make no representation or warranty of any kind regarding its accuracy, validity, reliability, or completeness. Consult with the appropriate professionals before taking any legal action. TemplateRoller.com will not be liable for loss or damage of any kind incurred as a result of using the information provided on the site.
TemplateRoller. All rights reserved. 2024 ©
Notice
This website or its third-party tools use cookies, which are necessary to its functioning and required to achieve the purposes illustrated in the cookie policy. If you want to know more or withdraw your consent to all or some of the cookies, please refer to the cookie policy.